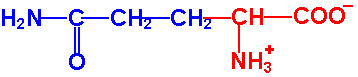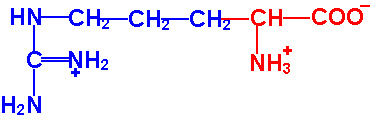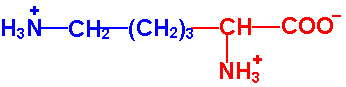Amino Acids and Proteins
Remember that all a protein consists of is a linear string of amino acids, similiar to the cars of a train. In the case of human proteins, there are only 20 different types of amino acids, or train cars, to choose from. In order to best understand amino acids and proteins, we can easily define the terms that are used to discuss the characteristics of amino acids and proteins. Do not let yourself be intimidated by these terms, even though they may be biochemical terms.
Let us first start by discussing an amino acid.
Each of the 20 different types of amino acids all have a common internal structure of carbon, hydrogen, oxygen, and nitrogen atoms. 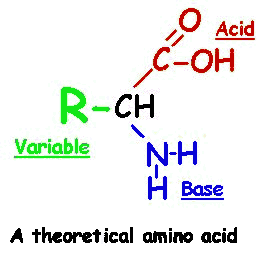
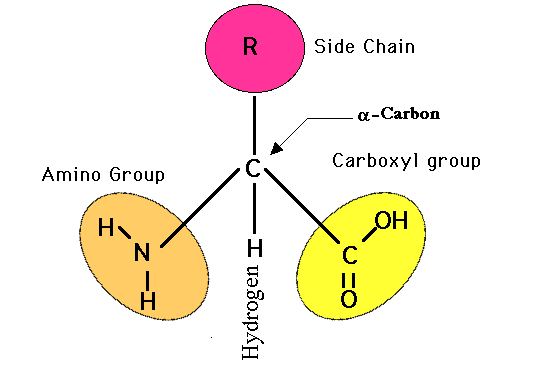 As you view the diagram, you will see that this internal structure consists of a central carbon atom with a nitrogen atom covalently bonded to it (by convention the nitrogen is drawn to the left of this central carbon atom) AND also covalently bonded to this same central carbon atom is another carbon atom (by convention drawn to the right of this central carbon atom). This I hope you will agree is quite a simple arrangement, a "nitrogen"--"central carbon"--"carbon" (N-C-C) structure. The nitrogen could not be any simpler in that along with its bond to the central carbon, it only has hydrogens attached to it. This nitrogen and its two hydrogens is referred to as an "amino" group. So, in other words, we have an amino group attached to the central carbon. This amino group attachment to the central carbon will be found in all 20 different amino acids.
As you view the diagram, you will see that this internal structure consists of a central carbon atom with a nitrogen atom covalently bonded to it (by convention the nitrogen is drawn to the left of this central carbon atom) AND also covalently bonded to this same central carbon atom is another carbon atom (by convention drawn to the right of this central carbon atom). This I hope you will agree is quite a simple arrangement, a "nitrogen"--"central carbon"--"carbon" (N-C-C) structure. The nitrogen could not be any simpler in that along with its bond to the central carbon, it only has hydrogens attached to it. This nitrogen and its two hydrogens is referred to as an "amino" group. So, in other words, we have an amino group attached to the central carbon. This amino group attachment to the central carbon will be found in all 20 different amino acids.
Now looking at the carbon that is attached to the right of the central carbon, it has attached to it an oxygen with a double bond and another oxygen with a hydrogen attached. This is commonly referred to as an acid or carboxyl group, this COOH group. We have an amino group and an acid group attached to this central carbon, hence an 'amino acid' molecule. You will also notice that this central carbon has a single hydrogen attached to it (shown by convention below the central carbon). If you click on the diagram showing all 20 different amino acids, you will see that all 20 different amino acids have this identicle internal structure.
Hopefully, now that you know what to look for, these 20 amino acids are not too complicated molecularly. In fact, the only thing that is different in these 20 amino acids is the group of atoms attached to the central carbon at this central carbon's fourth covalent bond (by convention, shown above the central carbon). For example, the simpliest amino acid only has a single hydrogen atom attached as this side group. Likewise, alanine only has a CH3 side group.
Typically the 20 different amino acids are then subdivided into groups according to the similiarities of there side groups. Some of the 20 different amino acids have a cyclic side groups attached (phenylalanine, tyrosine, tryptophan and histidine) and so they are grouped together into what is called the 'aromatic group'. Similarly, some side groups will act as acids in the pH of body fluids (remember that the human body blood pH is 7.4) and so they are grouped as the 'acidic amino acids'.
If you would look at this group for a minute, you'll notice that there is aspartic acid and asparagine (very similiar names) and glutamic acid and glutamine (again, very similiar names). If you look at their side groups, they are quite similiar in that the two acids (aspartic acid and glutamic acid) have the oxygen of the acid group (COOH) replaced with an amino group (NH2) and so it is no longer an acid group so that the word acid in their name is dropped and replaced with an 'ine' ending. I point this detail out to you so that you will not see these as a list of 20 aliens, but 20 friends that once you get to know a bit are easy to get along with. You only need to get to know them on their level, that is on a biochemical level.
Amino acid side chains are sorted into groups as follow :
In this group the side chain is aliphatic. An aliphatic group is hydrophobic.
 Glycine, Gly, G Glycine, Gly, G Alanine, Ala, A Alanine, Ala, A Valine, Val, V Valine, Val, V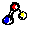 Leucine, Leu, L Leucine, Leu, L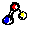 Isoleucine, Ile, I Isoleucine, Ile, I
|
In this group the side chain is an organic acid. The side chain is negatively charged.
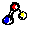 Aspartic Acid, Asp, D Aspartic Acid, Asp, D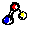 Glutamic acid, Glu, E Glutamic acid, Glu, E
In this group the side chain is an amide
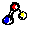 Asparagine, Asn, N, Asparagine, Asn, N,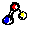 Glutamine, Gln, Q. Glutamine, Gln, Q.
|
In this group the side chain contains a sulphur. They are hydrophobic and cysteines are very reactive.
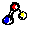 Methionine, Met, M Methionine, Met, M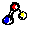 Cysteine, Cys, C Cysteine, Cys, C
|
In this group the side chain is an alcohol.
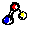 Serine, Ser, S Serine, Ser, S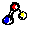 Threonine, Thr, T Threonine, Thr, T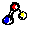 Tyrosine, Tyr, Y . Note, Tyrosine is also aromatic Tyrosine, Tyr, Y . Note, Tyrosine is also aromatic
|
In this group the side chain is an organic base. The side chain is hydrophilic.
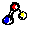 Arginine, Arg, R Arginine, Arg, R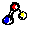 Lysine, Lys, K Lysine, Lys, K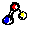 Histidine, His, H Histidine, His, H
|
In this group the side chain is aromatic. The side chain is very hydrophobic.
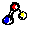 Phenylalanine, Phe, F Phenylalanine, Phe, F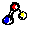 Tryptophan, Trp, W. Tryptophan, Trp, W.
In this group the side chain is an imine
. This amino acid is found in bends due to its structure.
 Proline, Pro, P. Proline, Pro, P.
|
Now you will need to use your imagination a bit. Keep in mind that these are real molecules in the real world with a 3-dimensional shape, not the 2-dimensional shape as they are drawn on the page. Try to imagine a pyramid with the central carbon hidden from view at the center of this pyramid. But branching out from this central carbon producing the four corners of the pyramid are the four things attached to the central carbon. One corner of the pyramid is the amino group (NH2), another corner of this pyramid is the acid group (COOH), another corner is the single hydrogen atom (H), and the fourth corner is the variable side group (commonly designated 'R-group').
That is how the amino acids really look in your body. Since there are four different things attached to the central carbon, this molecule is asymmetric. Imagine looking at an amino acid in the mirror. There is the central carbon with a group attached to it to the right, to the left, to the top, and to the bottom. The original amino acid and its mirror image are chemically the same, all the same pieces, but 3-dimensionally they are different, they have a different 3-dimensional arrangement of all the same atoms. It would be the same as looking at your left hand in the mirror. That image is just like your left hand, but if it were real it would not fit into a left hand glove. It is 3-dimensionally the opposite, even though it consists of all the same components.
In nature, we will find both the 'right-hand' and 'left-hand' form of all these 20 amino acids. The term that is used to distinguish between whether we have the 'right-hand' form or the 'left-hand' form are the letters "D" and "L". For reasons that are not completely understood, all of the amino acids that are used by organisms to make proteins are of the "L" form. In other words, all the amino acids that you have making up all of your proteins are the "L" form, L-serine, L-proline, etc.
It is also of interest to mention that there are several biologically important amino acids not found in human proteins. For example, the amino acids L-homoserine, L-ornithine and sarcosine are never used as part of a protein, but are found in our bodies as intermediate amino acids when converting one kind into another. Another important amino acid used in the human body, but not used directly for proteins is the amino acid gamma-aminobutyric acid (GABA) which is used as a neurotransmitter between neurons.
You will notice that the amino acid cysteine has a sulfur containing side group. Commonly if one cysteine becomes arranged across from another cysteine amino acid, the two cysteines will each lose a hydrogen atom from the sulfur and use these open bonds to covalently bond the two sulfur atoms and so covalently link the two cysteines together. In this form the amino acid is called cystine. So as a free amino acid it will be called cysteine since the sulfur will contain its hydrogen atom. However, when part of a protein chain, most likely the cysteine will link to another cysteine and now be called cystine.
To be complete, we should also mention that there are four amino acids that can be found in proteins that are slightly modified from their original structure. These are phosphoserine (serine plus a phosphate group), hydroxyproline (proline plus a hydroxy group (OH)), hydroxylysine (lysine plus a hydroxy group), and carboxyglutamic acid (glutamic acid plus an additional acid group (COOH)).
Protein Structure:
Now that we have discussed in good detail amino acids, we can now start to talk about linking them together, one by one, into a linear chain, similiar to the cars of a train, as we mentioned at the very beginning of this page.
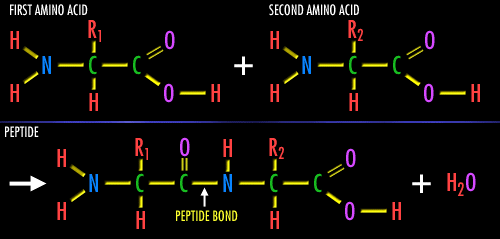
Joining Amino Acids Together: 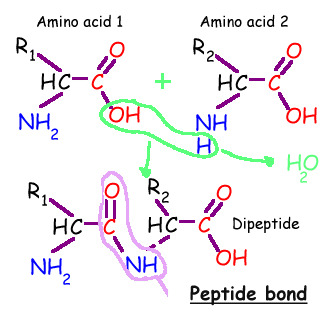
Individual amino acids may be linked together, one after another, like cars of a train. The covalent bond that is used to attach one amino acid to the next is called a 'peptide bond'. All that is done is that an -OH group is removed from the first amino acid and a -H is removed from the second amino acid being linked to the first. What is removed is -OH plus -H which can combine into H-O-H, or a water molecule. The exposed, broken bonds left on the two amino acids are then attached together, thus linking these two amino acids together, creating this peptide bond. This can be repeated again to attach another amino acid to the chain. Since the by-product of this reaction is simple water, these types of reactions are also called 'dehydration reactions'. This is how our cells build proteins. In fact, for us to break apart a protein, to digest a protein in the meat we eat, the enzymes in our intestines break this very same 'peptide bond', then break apart a water molecule into a -OH and a -H, then add back the -OH onto the one exposed bond on the first amino acid and add back the -H onto the second exposed bond from the broken peptide bond. In other words, just the opposite direction as our creating the peptide bond. That's it.
3-D Structure of a Protein:
Keep in mind that all proteins in the body are simply a linear chain of amino acids. This long chain of one amino acid after another and another however is not loose like an unwound piece of string. Instead, this long, linear string of amino acids is looped and coiled around into a three dimensional ball. Because every protein has its unique three dimensional shape, and this three dimensional shape essentially determines what that protein will be and what it will do, we need a way to describe its three dimensional shape with all its loops and folds. In order to do this, so that you could talk to someone over the telephone 1000's of miles away and describe in exact detail what your protein looks like, a series of terms has been created to describe every protein's three dimensional shape.
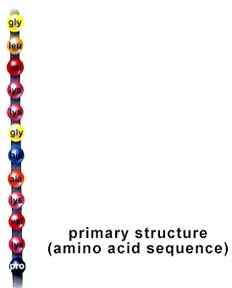
First the protein's Primary Structure
Second, the protein's Secondary Structure
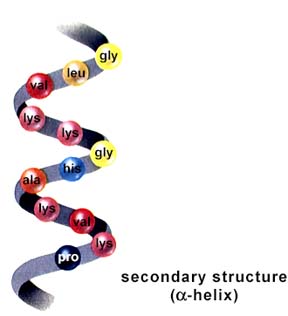
Thirdly, the protein's Tertiary Structure
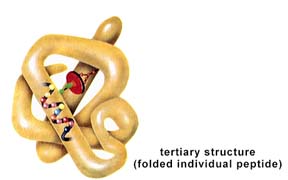
Lastly, the protein's Quaternary Structure

Now since there are weak bonds running across the looped and coiled string of protein to hold it together in its three dimensional shape, it is possible to break these small bonds. If these bonds are broken apart, we have then unraveled the coiled ball of protein. It will no longer have its three dimensional folding and looping, but instead will now start to take on a looser, uncoiled shape. You could even break all the small side bonds causing the entire string of amino acids to become just one long strand of protein. Remember, all we are doing is breaking the weak side bonds giving the protein its three dimensional coiled shape. We are not discussing the breaking of the strong, covalent bonds that hold each amino acid to its neighbor. This unraveling of the protein is called Denaturation
To denature a protein is to destroy its secondary, tertiary and quaternary structure, but you do not destroy its primary structure.
Where Do Proteins Come From?
All the proteins that you will find in a cell, or in your body are manufactured by a two step process of Protein Synthesis. The first step is called Transcription
The second step is called Translation
Protein in the diet

Table of a-Amino Acids Found in Proteins
| Amino Acid |
Symbol |
Structure* |
pK1 |
pK2 |
pK R Group |
| Amino Acids with Aliphatic R-Groups |
| Glycine |
Gly - G |
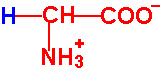 |
2.4 |
9.8 |
|
| Alanine |
Ala - A |
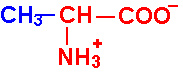 |
2.4 |
9.9 |
|
| Valine |
Val - V |
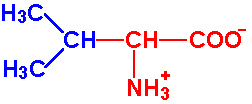 |
2.2 |
9.7 |
|
| Leucine |
Leu - L |
 |
2.3 |
9.7 |
|
| Isoleucine |
Ile - I |
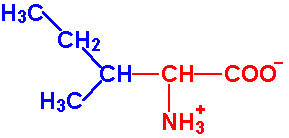 |
2.3 |
9.8 |
|
| Non-Aromatic Amino Acids with Hydroxyl R-Groups |
| Serine |
Ser - S |
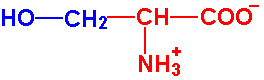 |
2.2 |
9.2 |
~13 |
| Threonine |
Thr - T |
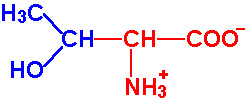 |
2.1 |
9.1 |
~13 |
| Amino Acids with Sulfur-Containing R-Groups |
| Cysteine |
Cys - C |
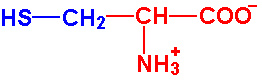 |
1.9 |
10.8 |
8.3 |
| Methionine |
Met-M |
 |
2.1 |
9.3 |
|
| Acidic Amino Acids and their Amides |
| Aspartic Acid |
Asp - D |
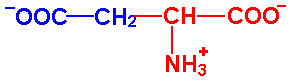 |
2.0 |
9.9 |
3.9 |
| Asparagine |
Asn - N |
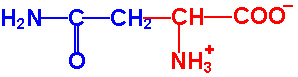 |
2.1 |
8.8 |
|
| Glutamic Acid |
Glu - E |
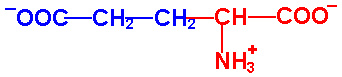 |
2.1 |
9.5 |
4.1 |
| Glutamine |
Gln - Q |
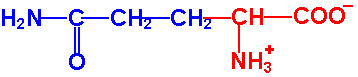 |
2.2 |
9.1 |
|
| Basic Amino Acids |
| Arginine |
Arg - R |
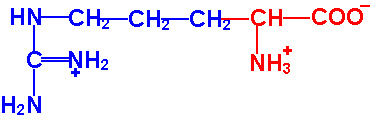 |
1.8 |
9.0 |
12.5 |
| Lysine |
Lys - K |
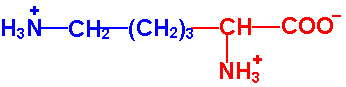 |
2.2 |
9.2 |
10.8 |
| Histidine |
His - H |
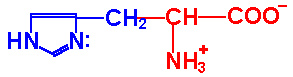 |
1.8 |
9.2 |
6.0 |
| Amino Acids with Aromatic Rings |
| Phenylalanine |
Phe - F |
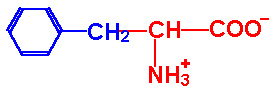 |
2.2 |
9.2 |
|
| Tyrosine |
Tyr - Y |
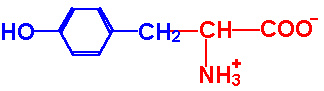 |
2.2 |
9.1 |
10.1 |
| Tryptophan |
Trp-W |
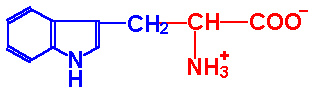 |
2.4 |
9.4 |
|
| Imino Acids |
| Proline |
Pro - P |
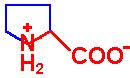 |
2.0 |
10.6 |
|
- *Backbone of the amino acids is red, R-groups are blue

 As you view the diagram, you will see that this internal structure consists of a central carbon atom with a nitrogen atom covalently bonded to it (by convention the nitrogen is drawn to the left of this central carbon atom) AND also covalently bonded to this same central carbon atom is another carbon atom (by convention drawn to the right of this central carbon atom). This I hope you will agree is quite a simple arrangement, a "nitrogen"--"central carbon"--"carbon" (N-C-C) structure. The nitrogen could not be any simpler in that along with its bond to the central carbon, it only has hydrogens attached to it. This nitrogen and its two hydrogens is referred to as an "amino" group. So, in other words, we have an amino group attached to the central carbon. This amino group attachment to the central carbon will be found in all 20 different amino acids.
As you view the diagram, you will see that this internal structure consists of a central carbon atom with a nitrogen atom covalently bonded to it (by convention the nitrogen is drawn to the left of this central carbon atom) AND also covalently bonded to this same central carbon atom is another carbon atom (by convention drawn to the right of this central carbon atom). This I hope you will agree is quite a simple arrangement, a "nitrogen"--"central carbon"--"carbon" (N-C-C) structure. The nitrogen could not be any simpler in that along with its bond to the central carbon, it only has hydrogens attached to it. This nitrogen and its two hydrogens is referred to as an "amino" group. So, in other words, we have an amino group attached to the central carbon. This amino group attachment to the central carbon will be found in all 20 different amino acids.